(Each and every questions are not answered, they will be added soon and in any chaos please comment)
1 Use of the Data Booklet is relevant to this question.
What could be the proton number of an element that has three unpaired electrons in each of its
atoms?
A 5 B 13 C 15 D 21
Solution:
To solve the question, you need to make the box diagrams of the above listed elements and hence fill it.
Since, the question asks you to give the element which have three unpaired electrons, we need to find out the half filled "p" orbital as it only can have three unpaired or half filled orbital i.e.
proton number 15=1s2 2s2 2p6 3s2 3p3 (box diagram will show you the three boxes of p orbital consist of only one electron in each
4 Some bond energy values are listed below.
bond bond energy/ kJ mol–1
C–H 410
C–Cl 340
Cl–C l 244
Br–Br 193
These bond energy values relate to the following four reactions.
P Br2 → 2Br
Q 2Cl → Cl2
R CH3 + C l → CH3Cl
S CH4 → CH3 + H
What is the order of enthalpy changes of these reactions from most negative to most positive?
A P → Q → R → S
B Q → R → S → P
C R → Q → P → S
D S → P → Q → R
solution:
bond breaking process is endothermic (positive value) and bond formation is exothermic (negative value). So, lets calculate the enthalpy changes of reaction P, Q, R, S.
P- Br-Br bond gets broken, hence, enthalpy change is +193 KJ/mol
Q- Cl-Cl bong is formed, so enthalpy change is -244 KJ/mol
R- C-Cl bond is formed, so enthalpy change is -340KJ/mol
S- C-H bond is broken hence enthalpy change is +410 KJ/mol
Now, lets sort this out and we get the order R<Q<P<S
answer is c
5 Given the following enthalpy changes,
I2 (g) + 3Cl2 (g) → 2ICl3 (s) ∆H = –214 kJ/mol
I2 (s) → I2 (g) ∆H = +38 kJ/mol
What is the standard enthalpy change of formation of iodine trichloride, ICl3 (s)?
A +176 kJ mol–1
B –88 kJ mol–1
C –176 kJ mol–1
D –214 kJ mol–1
solution:
first thing to do in this type of question is to draw the hess cycle so we too will do that here:
the equation will be
2ΔHf=(+38) + (-214)
=-176/2
=-88
the answer is B
6 Ammonium nitrate, NH4NO3 , can decompose explosively when heated.
NH4NO3 → N2O + 2H2O
What are the changes in the oxidation numbers of the two nitrogen atoms in NH4 NO3 when this
4 3
reaction proceeds?
A –2, –4 B +2, +6 C +4, –6 D +4, –4
solution:
note that NH4 NO3 is the ionic compound hence it charge is NH4 + and NO3- i.e. the oxidation number of nitrogen is -3 and +5 respectively.
and on reactant side the oxidation number of nitrogen is +1.
hence the change in oxidation no. is
+1-5=-4 and +1-(-3)=+4
so the answer is D
7 The Haber process for the manufacture of ammonia is represented by the following equation.
Which statement is correct about this reaction when the temperature is increased?
A Both forward and backward rates increase.
B The backward rate only increases.
C The forward rate only increases.
D There is no effect on the backward or forward rate.
solution:
Since, the reaction is exothermic it releases heat and and the temperature of system increases. According to Le Chatelier's principle, the equilibrium reaction strives to resist the change an hence to decrease the temperature the reaction shifts backward.
now note that the pressure is different, when the reaction shifts backward the pressure increases and this change is also resisted and the reaction shifts forward too.
so A is answer.
so A is answer.
8 Use of the Data Booklet is relevant to this question.
2.920 g of a Group II metal, X, reacts with an excess of chlorine to form 5.287 g of a compound
with formula XCl2 .
What is metal X?
A barium
B calcium
C magnesium
D strontium
solution:
1 mole of the metal reacts with excess of chlorine to produce one mole of the compound.
since, the no., of moles is same, it can be written as
n(X)=n(XCl2
or, 2.920/mr=5.287/(mr+71)
or, mr=87.6....which is strontium
so the answer is D
9 Which mass of gas would occupy a volume of 3 dm3 at 25 °C and 1 atmosphere pressure?
[1 mol of gas occupies 24 dm3 at 25 °C and 1 atmosphere pressure.]
A 3.2 g O2 gas
B 5.6 g N2 gas
C 8.0 g SO2 gas
D 11.0g CO2 gas
solution:
lets calculate the no. of moles of each compound and then multiply the answer by 24 (because 1 mole of gas occupies volume of 24dm3 at NTP.
you will get answer C
10 The table gives the concentrations and pH values of the aqueous solutions of two compounds, X
and Y. Either compound could be an acid or a base.
X Y
concentration 2 mol dm–3 2 mol dm–3
pH 6 9
Student P concluded that X is a strong acid.
Student Q concluded that the extent of dissociation is lower in X(aq) than in Y(aq).
Which of the students are correct?
A both P and Q
B neither P nor Q
C P only
D Q only
solution:
the pH of strong acid is less so P cant be true.
and the pH of base is high and since the pH is 9 it can comparatively stronger base which ,means its extent of dissociation is high. so Q is right.
hence the answer is D.
15 The percentage of ammonia obtainable, if equilibrium were established during the Haber process,
is plotted against the operating pressure for two temperatures, 400 °C and 500 °C.
Which diagram correctly represents the two graphs?

lets calculate the no. of moles of each compound and then multiply the answer by 24 (because 1 mole of gas occupies volume of 24dm3 at NTP.
you will get answer C
10 The table gives the concentrations and pH values of the aqueous solutions of two compounds, X
and Y. Either compound could be an acid or a base.
X Y
concentration 2 mol dm–3 2 mol dm–3
pH 6 9
Student P concluded that X is a strong acid.
Student Q concluded that the extent of dissociation is lower in X(aq) than in Y(aq).
Which of the students are correct?
A both P and Q
B neither P nor Q
C P only
D Q only
solution:
the pH of strong acid is less so P cant be true.
and the pH of base is high and since the pH is 9 it can comparatively stronger base which ,means its extent of dissociation is high. so Q is right.
hence the answer is D.
15 The percentage of ammonia obtainable, if equilibrium were established during the Haber process,
is plotted against the operating pressure for two temperatures, 400 °C and 500 °C.
Which diagram correctly represents the two graphs?

solution:
the chemical equation of Haber process is:
N2 + 3H2 ↔2NH3
When pressure is increased, the reaction resists the change and tries to oppose the change which can be done by forward reaction. hence, when pressure increases the reaction shifts forward and ammia yield increases. (c and d incorrect)
Haber process is exothermic so when temp decreases the reaction shifts forward to oppose the change and hence ammonia yield increases.
so answer is A.
24 What is formed when propanone is refluxed with a solution of NaBH4?
A propanal
B propan-1-ol
C propan-2-ol
D propane
solution:
ketones are formed when two degree alcohols are oxidised.
NaBH4 is the reducing agent. Hence, ketones when reacted with it gets reduced, the reaction is analogous to the backward oxidation reaction of ketones.
So, two degree alcohol is formed which is propan-2-ol.
answer is C

solution:
the given compound is ester. the -O- (of ester) bond gets broken and -OH gets added to the broken compound when ester reacts with H20.
so in this case the -O- bond is between C3H7 and CH3-C=O which are the decomposed compounds too.
and in C3H7, OH gets added and we get a product C3H7OH
B is the answer.

the chemical equation of Haber process is:
N2 + 3H2 ↔2NH3
When pressure is increased, the reaction resists the change and tries to oppose the change which can be done by forward reaction. hence, when pressure increases the reaction shifts forward and ammia yield increases. (c and d incorrect)
Haber process is exothermic so when temp decreases the reaction shifts forward to oppose the change and hence ammonia yield increases.
so answer is A.
24 What is formed when propanone is refluxed with a solution of NaBH4?
A propanal
B propan-1-ol
C propan-2-ol
D propane
solution:
ketones are formed when two degree alcohols are oxidised.
NaBH4 is the reducing agent. Hence, ketones when reacted with it gets reduced, the reaction is analogous to the backward oxidation reaction of ketones.
So, two degree alcohol is formed which is propan-2-ol.
answer is C

solution:
the given compound is ester. the -O- (of ester) bond gets broken and -OH gets added to the broken compound when ester reacts with H20.
so in this case the -O- bond is between C3H7 and CH3-C=O which are the decomposed compounds too.
and in C3H7, OH gets added and we get a product C3H7OH
B is the answer.

solution:
lets make a dispalyed formula of 1,1- Dichloroethene.
lets make a dispalyed formula of 1,1- Dichloroethene.

In polymerization, the C=C bond gets broken and the carbocation is the repeat unit for polymers.
the polymer with this repeat unit will look like:
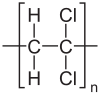



the polymer with this repeat unit will look like:




write the structural formula of the compound which is A.
Note: A and C look similar but in C the third and fourth carbon possess only 1 H and Cl which is incorrect.
Note: A and C look similar but in C the third and fourth carbon possess only 1 H and Cl which is incorrect.
